Abstract
Fanconi anemia (FA), a genetic disorder affecting DNA repair, is characterized by bone marrow failure and cancer susceptibility. In FA mouse models, metformin (N,N-dimethylguanide) a biguanide metabolic agent, improves blood counts and delays tumor development. Given these findings, we conducted a single institution pilot study of metformin in non-diabetic patients with FA to assess feasibility, tolerability, and impact of metformin on hematologic response as defined by modified MDS International Working Group (IWG) criteria (Table 1).
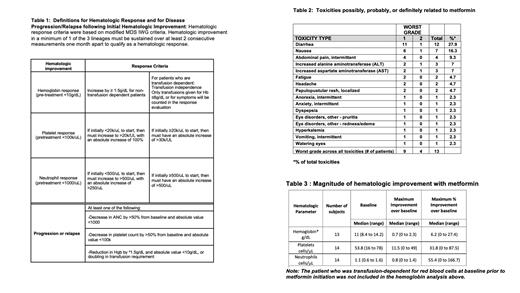
Fourteen of 15 patients with at least 1 cytopenia (hemoglobin <10g/dL, platelet count <100k/uL, or an ANC <1000 cells/uL) who signed treatment consent were eligible to receive metformin for 6 months; 1 patient was deemed ineligible given clonal disease progression on pretreatment bone marrow evaluation. Dosing of metformin was age based and equivalent to that used for type II diabetes mellitus. Of patients treated, the median patient age was 9.4 years (range 6.0-26.5), and 8/14 were male (57%). Median blood counts at study entry were hemoglobin of 11 g/dL (range 8.4 -14.2), platelet count of 54k/uL (range 16 -78), ANC of 1100 cells/uL (range 600 -1600). One patient was transfusion-dependent for red blood cells. Twelve of 14 patients (86%) completed 6 months of metformin treatment. One patient was lost to follow up after 2 weeks of metformin treatment and a second withdrew from the study after 3 months to proceed to hematopoietic stem cell transplant. Thirteen of 14 subjects (93%) tolerated maximal metformin daily dosing for age; one subject had a dose reduction for grade 2 gastrointestinal symptoms. No subjects developed hypoglycemia or metabolic acidosis. Three subjects had mildly elevated insulin levels consistent with mild insulin resistance prior to drug start; in 2/3 instances insulin levels normalized after metformin therapy. With metformin treatment, the most common adverse event observed was diarrhea (12/14, 85.7%); n=11 cases were grade 1 and n=1 was grade 2. Additional toxicities observed are outlined in Table 2. Hematologic response was observed in 4 of 13 evaluable patients (30.8%, 90% CI: [11.3, 57.3]) who received at least one month of treatment. The median time to response was 84.5 days (range 71-128 days). Responses were noted in neutrophils (n=3), platelets (n=1), and red blood cells (n=1). One individual with severe transfusion-dependent anemia became transfusion-independent after 1 month of treatment. The magnitude of hematologic improvement for the entire study cohort is outlined in Table 3. No subjects met criteria for disease progression or relapse during treatment. Bone marrow assessment of cellularity was not significantly different post therapy (median of change 0; interquartile range: -12.5- 30, p= 0.82) and clonal abnormalities noted at enrollment did not evolve with treatment. Interestingly, chromosomal breakage with MMC or DEB was observed to be lower following metformin in paired pre/post metformin samples from 8/10 and 7/10 individuals, respectively. Colony forming cell assays were performed pre/post metformin in 4 bone marrow samples derived from individuals who lacked protocol defined hematologic response. In 2 of 4 cases, increased myeloid and erythroid colony-forming cells were observed following metformin therapy. Exploratory proteomic assessment of plasma proteins pre and post metformin exposure identified downregulation of cytokine and cytokine receptor interaction pathways and inflammatory pathways and upregulation of insulin signaling pathways after completion of metformin. We conclude that metformin is safe and tolerable in non-diabetic patients with FA and may provide therapeutic benefit for a subset of patients with this marrow failure/cancer predisposition condition. Future efficacy endpoints should address the utility of metformin as both a prophylactic agent aimed at curtailing progression of bone marrow failure and as a preventive agent aimed at reducing cancer risk in FA. A phase II multicenter study is warranted to study whether the treatment is efficacious in a larger subset of patients and to further elucidate the signaling pathways and mechanism of action by which metformin may provide benefit in FA.
Pollard: Kura Oncology: Membership on an entity's Board of Directors or advisory committees; Syndax: Membership on an entity's Board of Directors or advisory committees. Furutani: Keros Therapeutics: Current Employment, Current equity holder in publicly-traded company. Esrick: bluebird bio: Consultancy. Nakano: Novartis: Consultancy. Thompson: Celgene: Consultancy, Research Funding; bluebird bio: Consultancy, Research Funding; Biomarin: Research Funding; Vertex: Research Funding; Graphite Bio: Research Funding; Baxalta: Research Funding; CRISPR Therapeutics: Research Funding; Novartis: Research Funding; Agios Pharmaceuticals: Consultancy; Beam Therapeutics: Consultancy; Global Blood Therapeutics: Current equity holder in publicly-traded company.
metformin- use in Fanconi anemia in an effort to improve hematopoiesis

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal